Hydrochloric acid- a chemical substance that is presented in the form of the interaction of water and hydrogen chloride. In its pure form, the acid has no color. The technical form of the acid has a yellowish tint, because it contains iron, chlorine and some other elements. Hydrochloric acid is used in many areas of human life. The fields of application of hydrochloric acid are very diverse. Let's consider them further.
The use of hydrochloric acid in industry
For example, the food industry uses acid as a food additive E507. This additive is used in the manufacturing process of vodka products, as well as various syrups. The use of hydrochloric acid in the food industry mainly plays the role of a regulator of the acid state of products. In metallurgy, technical hydrochloric acid is popular. It is used to clean metal before soldering or tinning. Etching and pickling in electroforming is not complete without the participation of hydrochloric acid. It creates an active environment for the above mentioned processes.
In order for the use of hydrochloric acid in industry not to create unnecessary problems, it is necessary to approach the choice of its type and concentration with responsibility.
The use of hydrochloric acid in everyday life
You probably have not thought about the composition of cleaning products that you use daily. Many of them contain hydrochloric acid. Use in everyday life, for the toilet: we use products that are highly acidic, which is why they should only be used with rubber gloves. This will keep your hands from getting irritated.
Housewives use a solution of hydrochloric acid at home. Using it as a stain remover helps get rid of rust or ink on clothes. Hydrochloric acid should be stored in glass containers out of the reach of small children. If hydrochloric acid has got on the skin or mucous membranes, it is necessary to immediately wash the affected area with running water. The use of hydrochloric acid at home should be carried out in compliance with safety rules. In addition to the ability to remove difficult stains from clothes, hydrochloric acid is used to combat scale. In order not to aggravate the situation when cleaning dirty dishes, it is recommended to use a certain concentration of acid. In these cases, inhibited hydrochloric acid is used, the use of which allows you to maintain the integrity of dishes made of fragile materials.
To keep your home and clothes clean, you must have hydrochloric acid at home. Use in everyday life should be extremely careful so as not to harm yourself and the things you interact with.
The use of hydrochloric acid in medicine
Hydrochloric acid is one of the components of human gastric juice. Therefore, in case of a decrease in its concentration, medications based on hydrochloric acid are prescribed. The use of hydrochloric acid in medicine plays a special role in the health of a person with diseases of the digestive tract. Due to the presence of a certain amount of hydrochloric acid in the gastric juice, the food is digested, and the microorganisms that enter the stomach die.
Hydrochloric acid is also used to treat specific skin diseases (warts). The use in folk medicine has become widespread: to improve digestion with low acidity of gastric juice, it is necessary to take hydrochloric acid preparations before eating. The use of hydrochloric acid (salts) helps in the fight against digestive disorders.
The use of hydrochloric acid in construction
Hydrochloric acid is used to improve the quality of many construction processes. For example, adding hydrochloric acid to a concrete mix increases its frost resistance. Also, the mixture hardens faster, and the masonry becomes more resistant to moisture. It is also known the use of hydrochloric acid in construction as a limestone cleaner. Red brick is cleaned of dirt and traces of building materials with a 10% hydrochloric acid solution. It is important to remember that not all types of bricks are affected by hydrochloric acid without damaging the structure of the building product. Therefore, you need to use only ten percent hydrochloric acid. A chemical solution of hydrochloric acid significantly saves finances, because other cleaners can cost ten times more.
The low cost does not make the use of hydrochloric acid less effective. Acid is used in many industries: from medical to construction. But, like all other acids, hydrochloric acid has the ability to irritate the skin, and high concentrations can lead to burns.
You can buy hydrochloric acid online by clicking on the link
Acids - as chemical compounds are relatively widely used in everyday life. According to the state of aggregation, acids can be crystalline substances, liquids and gaseous. All acids have a sour taste, in fact, it is because of the sour taste that they are called acids. acids have different Chemical properties: I interact with bases, basic oxides, have a destructive effect on many metals, harm the body when it gets on the surface or inside. Sulfuric, hydrochloric, acetic acids can cause severe burns, destroy tissues. Therefore, when working with acids, precautions must be taken. If acid gets on clothes or on the surface of the body, then it must be washed off very quickly with plenty of running water or neutralized with an ammonia solution (ammonia). And if the acid has got on a wooden, metal or other surface, then it is neutralized with lime, chalk or soda. Acids must be stored in a well-closed container out of the reach of children, and there must be a tag with the name of the acid on the container. Where, for what purposes are acids used in everyday life?
Hydrochloric acid dissolves metals well, including zinc, tin, iron, but does not interact with gold, silver and copper. Hydrochloric acid can be mixed with water in any ratio. It is used for cleaning enameled and faience sinks, toilet bowls, washbasins from limescale. It can be used to clean fabrics from rust stains, ink (a weak acid solution is being prepared). Hydrochloric acid destroys fabrics made from linen, cotton, rayon. Causes chemical burns on contact with skin
Sulfuric acid is a stronger acid than hydrochloric acid, concentrated carbonizes sugar, wood, cotton, wool and causes very deep skin burns. When preparing a solution of sulfuric acid, the following rule must be followed: Acid is poured into water in a thin stream along the wall of a glass dish, but not vice versa. Sulfuric acid, due to its ability to absorb large amounts of water, is used to drain windows in the winter, after placing a concentrated solution between the frames sulfuric acid in a glass, filling 1/5 of the volume. Sulfuric acid is also used to prepare battery acid.
Nitric acid in everyday life is used only in the form of dilute solutions for cleaning products, primarily from precious metals.
Acetic acid it is used either in the form of table vinegar, with an acid concentration of up to 9%, or in the form of an 80 percent essence.
Diluted acid does not affect metals, tissues of plant and animal origin, human and animal integuments. Vinegar is used as a seasoning for dishes to reduce water hardness, remove fruit stains from fabrics.
Oxalic acid used to remove stains from ink, paint, rust. Crystalline acid can cause burns to the mucous membranes of the mouth, esophagus and stomach. It is a toxic substance.
Formic acid similar to vinegar, but poisonous, causing very severe burns and skin irritation.
Lemon acid-crystalline colorless substance, highly soluble in water and ethyl alcohol. It is used to remove all kinds of stains: from wine, various berries, paints, rust, ink.
Boric acid - a colorless crystalline substance, in medicine (boric ointment), as a microfertilizer and a means to combat cockroaches and house ants.
There are two types of acids: organic and inorganic, the differences between them are that the former always contain carbon molecules.
Organic acids enter the body with berries, vegetables, fruits and dairy products. Some acids are vitamins, such as vitamin C - ascorbic acid.
Inorganic acids can also come from food, but can also be produced by the body on its own. Hydrochloric acid is present in the gastric juice, under its action bacteria that enter the stomach with food die. Hydrosulfuric acid is found in mineral water.
Application of acids
Sulfuric acid ranks first among acids. It is used in large quantities for the production of fertilizers, chemical fibers, plastics, medicines. It is filled with acid batteries, used to extract metals from ore. In the oil industry, it is used to purify petroleum products.
Acetic acid has a bactericidal effect, its solution is used in food preservation, for the production of medicines, in the production of acetone, in dyeing and printing.
Hydrochloric acid is used to treat well zones in the oil industry.
Nitric acid plays an important role in the production of fertilizers, varnishes, dyes, plastics, explosives and medicines.
Phosphoric acid is included in degreasing compositions for metal materials before applying protective compositions to them. It is incorporated into rust-converting agents prior to paint application and used as corrosion protection for pipelines.
Citric acid is used in the creation of cosmetics, as a diluent and preservative. Due to its bleaching, cleansing and astringent properties, it is used in cleansing creams, hair rinses, anti-pigmentation creams, and hair dyes.
Acetylsalicylic acid is effective in the prevention of diseases of the cardiovascular system, reduces the formation of blood clots, has an analgesic effect, therefore it is intensively used in medicine.
Boric acid is also used in medicine for its antiseptic properties. It is used for pediculosis (lice), in the treatment of otitis, conjunctivitis, inflammation of the skin.
Stearic acid is used in soap making. Adding it to soap ensures that the product leaves the skin smooth, soft and has a soothing effect.
COURSE WORK
HYDROCHLORIC ACID. COMMODITY
Introduction
1. Application of hydrochloric acid in the field of production or consumption
3. Technology for the production of hydrochloric acid and its feasibility study
4. Standards for hydrochloric acid, normalized quality indicators in accordance with the requirements of standards
5. Quality control of goods. Standards for the rules for acceptance, testing, storage and operation of goods
5.2.1 Marking
5.2.2 Packaging
5.2.3 Safety requirements
5.2.4 Acceptance
5.2.5 Methods of analysis
Conclusion
List of sources used
Introduction
The volume of production at the enterprises of the chemical and petrochemical industry in Belarus in January-July this year increased in comparable prices by 5.4% compared to the corresponding period last year and amounted to about Br3.7 trillion.
For successful work in this field, it is necessary to constantly monitor the quality of products, as well as improve their quality characteristics.
Hydrochloric acid is the most important product of the chemical industry and is a colorless liquid with a pungent odor of hydrogen chloride.
aim term paper is an analysis of consumer properties and applications of hydrochloric acid.
There are a number of tasks to complete during the course of the work.
Consider the use of hydrochloric acid in the field of production and consumption;
Determine the classification features of hydrochloric acid;
Analyze the production technology of hydrochloric acid;
Consider quality indicators;
To identify standards for the rules for acceptance, testing, storage of hydrochloric acid.
The object of the course work is hydrochloric acid.
During the work, we used study guides on commodity science of non-food products of such authors as Epifantseva V.V., Sytsko V.E., Karolkova R.V. and other authors, as well as textbooks on the chemical industry, as well as the media.
Course work consists of five chapters, conclusion-conclusion, as well as a list of sources used.
1. Application of hydrochloric acid in the field of production or consumption
Shipment is made by railway tanks, in polyethylene barrels with a capacity of 20-45 3, in polyethylene canisters with a capacity of 20, 40 and 50 dm3.
By the way, known fact that hydrochloric acid is found in gastric juice (about 0.3%) and plays an important role, as it promotes the digestion of food and kills various pathogenic bacteria (cholera, typhoid, etc.). If the latter enter the stomach along with a large amount of water, then due to the dilution of the HCl solution, they survive and cause disease in the body. Therefore, during epidemics, raw water is especially dangerous. With an increase in the concentration of HCl in the stomach, "heartburn" is felt, which is eliminated by ingesting a small amount of NaHCO 3 or MgO. On the contrary, with insufficient acidity of gastric juice, hydrochloric acid is prescribed for oral administration (5-15 drops of 8.3% HCl per 1/2 cup of water before or during meals).
Production hydrochloric acid synthetic was mastered in 1962. During the period of operation, repairs were carried out, technological equipment was improved. The high quality of raw materials allows to obtain high quality acid. hydrochloric acid also used in the production of plastics, pesticides, intermediates and dyes for cleaning the surface of metals from oxides, carbonates, in the electrical and textile industries.
Hydrochloric acid inhibited Grade A - used for acid treatment of wells in the oil industry in order to improve the connectivity of wells with the formation (for expanding and cleaning pores and cracks, removing the filtration resistance of a reservoir composed of carbonate rocks-dolomites and limestones, or contaminated with carbonate deposits).
hydrochloric acid grade B - used for pickling ferrous and some non-ferrous metals and products from them, for chemical cleaning of boilers and apparatus from inorganic deposits.
Hydrochloric acid is used to obtain chlorides of Zn, Ba. Mg, Ca, Fe, A1, etc., for pickling in soldering and tinning, and non-ferrous metallurgy (extraction of Pt, An), in the hydrolysis of wood, in the production of dyes, for the hydrochlorination of organic compounds, etc.
2. Classification features of hydrochloric acid
Hydrochloric acid is produced in two grades: A and B.
According to physical and chemical indicators, technical synthetic hydrochloric acid must comply with the standards specified in Table 2. 1.
Table 2.1 Norms for hydrochloric acid.
| State name | Norm for the brand | Analysis Methods | ||
| AOCP 21 2211 0100 | BOKP21 2211 0200 | |||
| 1. Appearance | clear yellow liquid | According to 6.4 | ||
| 35 | 33 | 31,5 | According to 6.5 | |
| 0,001 | 0,002 | 0,015 | According to 6.6 | |
| 0,010 | 0,015 | 0,100 | According to 6.7 | |
| 0,002 | 0,002 | 0,008 | According to 6.8 | |
| 0,0001 | 0,0001 | 0,0002 | According to 6.9 | |
| 0,0003 | 0,0004 | 0,0005 | By 6.10 | |
The mass fraction of mercury is normalized in the acid obtained from hydrogen and chlorine by mercury electrolysis. It is allowed for the food industry, in agreement with the consumer, to manufacture acid with a mass fraction of hydrogen chloride of not more than 26%.
In the acid supplied for etching metals, the mass fraction of iron and residue after calcination is not standardized.
By agreement with the consumer, a mass fraction of hydrogen chloride of at least 30% is allowed in both grades of acid.
Hydrochloric acid is produced in the following grades: technical (27.5% HC1); synthetic (31% HC1), inhibitory (20% HC1) and reactive (35-38% HC1, density at 20°C is 1.17-1.19 g/cm3).
3. Technology for the production of hydrochloric acid and its feasibility study
sulfate;
Synthetic
However, it should be noted that the first two methods are losing their industrial significance.
The production of hydrochloric acid (reactive, sulphate-produced, synthetic exhaust) consists in the production of HCI followed by its absorption with water. Depending on the method of removing the heat of absorption, which reaches 72.8 kJ/mol, the processes are divided into isothermal (at a constant temperature), adiabatic (without heat exchange with environment) and combined.
1. The sulfate method is based on the interaction of sodium chloride NaCl with concentrated sulfuric acid H2SO4 at 500-550*C. Reaction gases from muffle furnaces contain 50-65% hydrogen chloride, and gases from fluidized bed reactors up to 5% HCI. At present, it is proposed to replace sulfuric acid with a mixture of SO2 and O2 using Fe2O3 as a catalyst and carrying out the process at a temperature of 540*.
2. The direct synthesis of hydrochloric acid is based on a combustion chain reaction:
Р2-CI2+2HCI +184.7 kJ (3.1)
The reaction is initiated by light, moisture, solid porous substances (charcoal, porous platinum) and some mineral substances (quartz, clay). Synthesis in combustion chambers is carried out with an excess of 5-10% H2. The chambers are made of steel, graphite, quartz, refractory bricks. The most modern material that prevents contamination of the product is graphite impregnated with phenol-formaldehyde resins. To prevent the explosive nature of combustion, the reagents are mixed directly in the flame of the burner. In the upper zone of the combustion chambers, heat exchangers are installed to cool the reaction gases to 150-160*C. The capacity of modern graphite furnaces reaches 65 tons/day (in terms of hydrochloric acid containing 35% HCI). In the case of hydrogen deficiency, various modifications of the process are used. For example, a mixture of CI2 with water vapor is passed through a layer of porous hot coal:
CO+H2O+CI2=2HCI+CO2 (3.2)
More than 90% of hydrochloric acid in the CIS is currently obtained from off-gas hydrogen chloride HCI, which is formed during the chlorination and dehydrochlorination of organic compounds, pyrolysis of organochlorine wastes, metal chlorides, production of potash non-chlorinated fertilizers, etc.
Off-gas gases contain various amounts of hydrogen chloride, inert impurities (N2H2CH4), organic substances poorly soluble in water (chlorobenzene, chloromethanes), water-soluble substances (acetic acid, chloral), acidic impurities and water.
HCI in exhaust gases. The most promising are absorbers that allow you to extract from the original off-gas from 65-85% HCI.
In industry, adiabatic absorption schemes are most widely used to produce hydrochloric acid. Off-gas gases are introduced into the lower part of the absorber, and water (or dilute hydrochloric acid) is introduced countercurrently into the upper part.
Hydrochloric acid is heated to boiling point by the dissolution temperature of HCI. The dependence of the change in absorption temperature and HCI concentration is shown in fig. 3.1
Rice . 3.1 Temperature distribution (curve 1) and concentration (curve 2) of HCI at the height of the adiabatic absorber
The absorption temperature is determined by the boiling point of the acid of the corresponding concentration, the maximum boiling point of the azeotropic mixture is about 110 *.
A typical diagram of the adiabatic absorption of HCI from off-gases from chlorination is shown in Figure 4.2. Hydrogen chloride is absorbed in the absorber 1, and the remains of poorly water-soluble organic substances are separated from the water after condensation in the apparatus 2, cleaned in the tail column 4 and separators 3.5 and commercial hydrochloric acid is obtained.
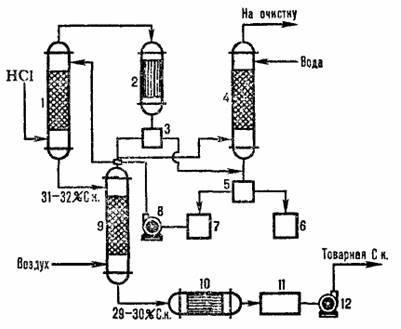
1 - abiabatic absorber; 2 - capacitor; 3, 5 - separators; 4 – tail column; 6 – organic phase collector; water phase collector; 8.12 - pumps; 9 – stripping column; 10 - heat exchanger, 11 - commercial acid collector.
Fig.3.2 Scheme of a typical adiabatic absorption of hydrochloric acid from waste gases
The production of hydrochloric acid from exhaust gases using a combined absorption scheme is presented in the form of a typical scheme in Fig. 3.3.
In the adiabatic absorption column, hydrochloric acid is obtained at a reduced concentration, but free from organic impurities. Acid with an increased concentration of HCI is produced in an isothermal absorption column at a reduced temperature. The degree of extraction of HCI from waste gases when dilute acids are used as absorbents is 90-95%. When pure water is used as the absorbent, recovery is nearly complete.
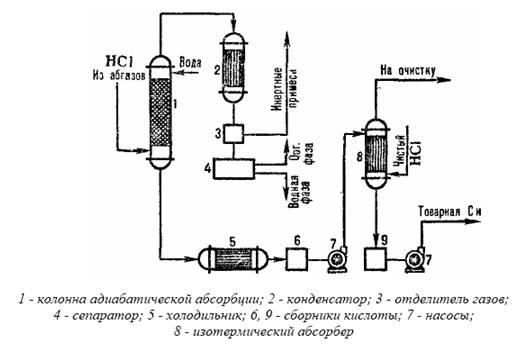
Fig.3.3 Scheme of a typical combined absorption of hydrochloric acid from off-gases
Hydrogen chloride is used for the production of organochlorine products by hydrochlorination of organic compounds, such as ethylene, acetylene.
4. Standards for hydrochloric acid, normalized quality indicators in accordance with the requirements of standards
GOST is a state quality standard.
Hydrochloric acid must comply with the following GOSTs:
GOST. 14261-77. Hydrochloric acid of special purity. Specifications. Date of introduction 01-07-1978. Date of change - 12/01/1987.
This standard applies to high purity hydrochloric acid, which is a colorless transparent liquid fuming in air.
GOST 30553-98. Acid hydrochloric technical. Determination of total acidity by titrimetric method. MKS code 71.060.30 Introduction date 01.01.2001. Establishes a titrimetric method for determining the total acidity (hydrogen chloride content) in commercial hydrochloric acid.
GOST 30554-98 Acid hydrochloric technical. Determination of sulfate ash by the gravimetric method. Date of introduction 01-07-2003.
GOST 30582-98. Acid hydrochloric technical. Determination of iron content by spectrometric method using 2,2"-bipyridyl. Introduction date 01-07-2003.
GOST 30621-98. Acid hydrochloric technical. Determination of arsenic content by photometric method using silver diethyldithiocarbamate. Date of introduction 01-07-2003.
GOST 3118-77 Reagents. Hydrochloric acid. Specifications. The standard applies to hydrochloric acid (an aqueous solution of hydrogen chloride), which is a colorless liquid with a pungent odor, fuming in air; miscible with water, benzene and ether.
GOST 857-95. Acid hydrochloric synthetic technical. Specifications. The standard applies to technical synthetic hydrochloric acid, obtained by absorption of hydrogen chloride by water, which is formed by the interaction of evaporated, electrolytic chlorine, off-gases from the liquefaction of chlorine with hydrogen.
5. Quality control of goods. Standards for the rules for acceptance, testing, storage and operation of goods
5.1 Theoretical foundations of quality and product standardization
STANDARD (from the English standard - norm, sample), in the broad sense of the word - a sample, standard, model, taken as initial for comparing other similar objects with them. The standard as a regulatory and technical document establishes a set of norms, rules, requirements for the object of standardization. The standard can be developed both for material objects (products, standards, samples of substances), and for norms, rules, requirements in various fields.
TECHNICAL CONDITIONS (TS) is a regulatory and technical document that establishes a set of requirements for products of specific types, brands, articles. Developed based on relevant standards.
A TECHNOLOGICAL CARD is a form of technological documentation that records the entire process of processing a product, indicates operations and their components, materials, production equipment and technological modes, the time required to manufacture a product, the qualifications of workers, etc.
ROUTE SHEET (map) is a document that records the production of products and the movement of a batch of machined parts through operations.
TECHNICAL REGULATIONS - a document containing mandatory requirements of society, approved by the competent government authority. For timber, technical regulations include documents containing requirements for radiation and phytosanitary safety, as well as the safety of transportation and processing.
The quality of a product (work, service) is a set of characteristics of a product (work, service) related to its ability to satisfy the established and (or) expected needs of the consumer (safety, functional suitability, operational characteristics, reliability, economic, informational and aesthetic requirements, etc.).
The complex of implemented measures in the field of quality includes the development and production of new products, the introduction of resource-saving technologies, as well as the reconstruction and technical re-equipment of production facilities.
Quality certificate - a written document or brand of a recognized refiner, which indicates the name of the precious metal, its sample, serial number and the name of the manufacturer's company
The Technical Code establishes the rules for the development, including approval, state registration of technical regulations, as well as the rules for their verification, revision, amendment, cancellation, application, official publication, notification of the progress of development and publication of information on technical regulations.
The state standard is one of the main categories of standards in the Republic of Belarus.
The integration of the Republic of Belarus into the world economy, the intensification of foreign economic activity, the promotion of Belarusian products to international markets, as well as the tasks of the country's socio-economic development have necessitated the reform of technical legislation.
In 2004, the Law of the Republic of Belarus "On technical regulation and standardization" was adopted and entered into force, which is based on the provisions of the World Trade Organization Agreements, takes into account aspects of the technical regulation and standardization systems of Russia, Ukraine and other countries, as well as the European Union.
5.2 Quality control of hydrochloric acid. Technical requirements
Hydrochloric acid is produced in two grades: A and B. According to physical and chemical parameters, technical synthetic hydrochloric acid must comply with the standards specified in table 5.1.
Table 5.1 Norms of hydrochloric acid
| State name | Norm for the brand | Analysis Methods | ||
| AOCP 21 2211 0100 | BOKP21 2211 0200 | |||
| premium OKP 21 2211 0220 | first grade OKP 21 2211 0230 | |||
| 1. Appearance | Clear colorless or yellowish liquid | clear yellow liquid | According to 6.4 | |
| 2. Mass fraction of hydrogen chloride, %, not less than | 35 | 33 | 31,5 | According to 6.5 |
| 3. Mass fraction of iron (Fe),%, no more | 0,001 | 0,002 | 0,015 | According to 6.6 |
| 4. Mass fraction of the residue after calcination,%, no more | 0,010 | 0,015 | 0,100 | According to 6.7 |
| 5. Mass fraction of free chlorine,%, no more | 0,002 | 0,002 | 0,008 | According to 6.8 |
| 6. Mass fraction of arsenic (As),%, no more | 0,0001 | 0,0001 | 0,0002 | According to 6.9 |
| 7. Mass fraction of mercury (Hg), % no more | 0,0003 | 0,0004 | 0,0005 | By 6.10 |
The appearance is determined visually in the transmitted light of a column of liquid poured into a cylinder 1.2-100 according to GOST 1770.
The determination method is based on the neutralization reaction of hydrogen ions with sodium hydroxide:
(5.1)
Methyl orange is used as an indicator.
Equipment, reagents, solutions:
Burette 1, 25 3-25-0.1 according to GOST 29251;
Flask Kn-1,2-100, 250-1 according to GOST 25336;
Flask 2-250, 1000 according to GOST 1770;
Pipette 2-20 according to GOST 29169;
Cylinder 1.2-25 according to GOST 1770;
Sodium hydroxide according to GOST 4328, chemically pure, concentration solution c(NaON) = 0.1 mol/dm3, prepared according to GOST 25794.1;
Methyl orange (indicator), solution with a mass fraction of 0.1%; an aqueous solution is prepared according to GOST 4919.1;
Distilled water according to GOST 6709 and not containing carbon dioxide is prepared according to GOST 4517.
In a pre-weighed flask with a ground stopper with a capacity of 100 cm 3 and containing 20 cm 3 of water, 3 cm 3 of the analyzed acid are placed and weighed again (the weighing result is recorded to the fourth decimal place). The solution is quantitatively transferred into a volumetric flask with a capacity of 250 cm 3, repeatedly rinsed with distilled water, poured into a volumetric flask, the volume is adjusted to the mark with water and mixed. Pipette 20 cm 3 of the resulting hydrochloric acid solution into a conical flask with a capacity of 250 cm 3, add 25 cm 3 of water, 2-3 drops of a methyl orange indicator and titrate with sodium hydroxide solution until the red color changes to yellow.
Mass fraction of hydrogen chloride X , %, calculated by the formula:
Where V - the volume of sodium hydroxide solution with a concentration exactly c (NaOH) = 0.1 mol / dm 3, which went for titration, cm 3;
V 1 - the volume of the solution of the analyzed hydrochloric acid, taken to perform the analysis, cm 3;
m - mass of the flask with water, g;
m 1
- mass of the flask with water and analyzed acid, g;
0.003646 - mass of hydrogen chloride, corresponding to 1 cm 3 sodium hydroxide solution, concentration exactly c (NaOH) \u003d 0.1 mol / dm 3, g / cm 3.
The result of the analysis is taken as the arithmetic mean of the results of two parallel measurements, the allowable discrepancies between which should not exceed 0.3% with a confidence level P = 0.95 .
Permissible discrepancies between the results obtained in the two laboratories should not exceed 0.6%. Relative total error of determination ±2% at confidence level Р= 0.95 .
The mass fraction of iron is determined in hydrochloric acid after diluting the sample without its preliminary neutralization. Neutralization is carried out after the introduction of sulfosalic acid, that is, neutralization and the formation of a sulfosalicylate complex of iron 3, colored yellow in a slightly alkaline medium (pH8.0-11.5), take place simultaneously. The light absorption intensity of the formed complex is measured on a photoelectric colorimeter. Measurement range 5·10 -4 - 2.0·10 -2%.
Equipment, solutions, reagents:
Photoelectric laboratory colorimeter FEK-56M, KFK or other type, providing the specified sensitivity and accuracy;
Stopwatch mechanical any brand;
Cup SZ-14/8 according to GOST 25336;
Flasks 1.2-50, 100, 250 and 1000 cm 3 according to GOST 1770;
Pipettes 1, 2, 5, 7-1, 25, 2, 5, 10 according to GOST 29169;
Hydrochloric acid according to GOST 3118, chemically pure, aqueous solution (1:1);
Aqueous ammonia according to GOST 3760, analytical grade, solution with a mass fraction of 25%;
Sulfosalicylic acid according to GOST 4478, analytical grade, concentration solution 100 g/dm 3 ;
Iron-ammonium alum in accordance with the current regulatory documentation;
A solution of iron with a concentration of 1 mg/cm 3 is prepared according to GOST 4212, a freshly prepared solution of a concentration of 10 μg/cm 3 is prepared by dilution;
Distilled water according to GOST 6709.
Preparation for analysis
Preparation of a solution of sulfosalic acid 10 g of sulfosalic acid is transferred into a volumetric flask with a capacity of 100 cm 3, dissolved, the volume is adjusted to the mark with water, mixed. Weighing results are recorded to the second decimal place.
Preparation of calibration solutions and calibration of the photoelectric colorimeter.
Graduation and determination is carried out according to GOST 10555 by the sulfosalicylic method.
30 cm 3 of distilled water are introduced into volumetric flasks with a capacity of 50 cm 3, 1 cm 3 of hydrochloric acid solution, 1.0, is added with a pipette; 2.0; 3.0; 4.0; 6.0 cm 3 of a solution of iron with a concentration of 10 μg / cm 3, 2 cm 3 of a solution of sulfosalic acid and 5 cm 3 of a solution of ammonia. After adding each reagent, the solution is stirred. The volume of the solution was made up to the mark with water and mixed. At the same time, a control solution is prepared: 30 cm 3 of water, 1 cm 3 of hydrochloric acid are introduced into a volumetric flask with a capacity of 50 cm 3, 2 cm 3 of a solution of sulfosalicylic acid are added, and then proceed as described above.
The optical density of the calibration solutions is measured after (10 ± 1) min in cuvettes with a light-absorbing solution layer thickness of 50 mm at a wavelength of 434 nm relative to the control solution. It is allowed to calibrate the instrument using the least squares method.
Based on the results obtained, a calibration graph is built, plotting the mass of iron introduced into the calibration solutions in micrograms along the abscissa axis, and the corresponding values of optical densities on the ordinate axis. The calibration curve is checked once a quarter, as well as when replacing reagents or instruments.
Conducting an analysis
A portion of the analyzed hydrochloric acid weighing (20 ± 1) g is quantitatively transferred into a volumetric flask with a capacity of 250 cm 3, rinsing the glass several times with water, adjusting the volume of the solution with water to the mark and mixing. Weighing results are recorded to the second decimal place.
For grades A and B of the highest grade, 25 cm 3 are taken with a pipette, and for grade B of the 1st grade - 2.5 cm 3 of the prepared solution, transferred to a volumetric flask with a capacity of 50 cm 3, add 2 cm 3 of a solution of sulfosalicylic acid and mix. Then add 10 cm 3 of ammonia solution, bring the volume to the mark with water and mix.
Prepare the control solution as described in 6.6.3.2. After (10 ± 1) min, the optical density is measured and, using the calibration graph, the mass of iron in the analyzed solution is found in micrograms.
Results processing
Mass fraction of iron X 1 %, calculated by the formula:
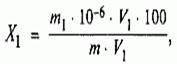
Where m 1 is the mass of iron in the analyzed solution, found from the calibration curve, μg;
m - weight of the sample of hydrochloric acid, taken to perform the analysis, g;
V - the volume of hydrochloric acid solution obtained after diluting the mass of hydrochloric acid, cm 3;
V 1 - volume of dilute hydrochloric acid solution taken for analysis, cm 3 .
The result of the analysis is taken as the arithmetic mean of the results of two parallel measurements, the discrepancies between which should not exceed 0.0005%. The results of the determination are rounded to the fourth decimal place.
Permissible discrepancies between the results obtained in the two laboratories should not exceed 0.0005%. The absolute total error of the determination is ±0.2 A, where A is the result of the determination at a confidence level P = 0.95 .
Determination of the mass fraction of the residue after calcination
The mass fraction of the residue after calcination at 600 °C is measured by the gravimetric method. The determination range is from 0.005% to 0.100%.
Equipment, solutions and reagents:
Cylinder 2-100 according to GOST 1770;
KP-type quartz cup with a capacity of 100 cm 3 according to GOST 1990S, platinum or porcelain;
Desiccator 2-190 mm, 250 mm according to GOST 25336;
A muffle furnace with a thermocouple that maintains a temperature of (600±10) °C;
Sulfuric acid according to GOST 4204, chl.;
Calcium chloride, calcined at 250-300 °C;
Distilled water according to GOST 6709;
Hourglass for 5 min.
Preparation for analysis
The cup is calcined in a muffle furnace at a temperature of (600 ± 10) °C for (5 ± 1) minutes. Then the cup is placed in a desiccator with calcium chloride and incubated for (30 ± 5) min. The chilled cup is weighed. Weighing results are recorded to the fourth decimal place.
Conducting an analysis
85 cm 3 of hydrochloric acid to be analyzed is taken with a cylinder and placed in a quartz cup, 1 drop of sulfuric acid is added and the mixture is evaporated almost to dryness on a water bath. The cup with the residue is heated on an electric stove until the emission of sulfuric acid vapors ceases. Evaporation of the analyzed acid and decomposition of sulfuric acid can be carried out under an infrared lamp.
After that, the cup with the residue is transferred to a muffle furnace preheated to (600 ± 10) °C and calcined for (5 ± 1) min. Transfer the cup to a desiccator, stand (30 ± 5) min and weigh.
Results processing
Mass fraction of residue after calcination X 2 %, calculated by the formula:
Where m 1 - mass of the cup with the residue after calcination, g;
m - weight of the empty cup, g;
V - the volume of the hydrochloric acid sample taken for analysis, cm 3;
p - density of hydrochloric acid, g/cm 3 .
The result of the analysis is taken as the arithmetic mean of the results of two parallel measurements, the allowable discrepancies between which should not exceed 0.0006%. The results of parallel determinations are rounded up to 0.0001%, the result of the determination is 0.001%. Permissible discrepancies between the results obtained in the two laboratories should not exceed 0.0008%. Absolute total error of determination ± 0.0005% at confidence level P = 0.95 .
5.2.6 Determination of the mass fraction of free chlorine
The method is based on the oxidation reaction of methyl orange with chlorine:
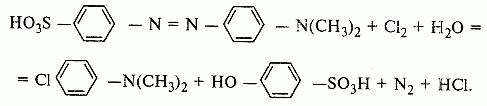 |
(6.5)
As a result of the oxidation of methyl orange, the color of its solutions becomes less intense. The intensity of the color depends on the order of mixing the solutions, so the analyzed hydrochloric acid is added last with stirring. The method is selective, iron (III) interferes with the determination. Measurement range 5·10 -4 - 8·10 -3%.
Equipment, reagents and solutions:
Photoelectric laboratory colorimeter KFK or other type, providing the specified sensitivity and accuracy;
Volumetric flasks 2-25, 1000 according to GOST 1770;
Pipettes 1.5-1.2 according to GOST 29169;
Hydrochloric acid according to GOST 3118, chemically pure, solution (1:2);
Methyl orange (indicator), a solution with a concentration of 0.1 g / dm 3, is prepared as follows: 0.1 g of methyl orange is transferred into a volumetric flask with a capacity of 1000 cm 3, the volume of the solution is adjusted to the mark with water and mixed. Weighing results are recorded to the second decimal place.
Preparation of calibration solutions and calibration of the photoelectrocolorimeter
15 cm 3 of water are introduced into volumetric flasks with a capacity of 25 cm 3, 2.0 is added with a pipette; 1.6; 1.2; 0.8; 0.4 cm 3 solution of methyl orange, which corresponds to 0; 10; 20; thirty; 40 μg of chlorine, add 1 cm 3 of hydrochloric acid solution, dilute the volume of the solution with water to the mark and mix.
The optical density of the obtained solutions is measured on a photoelectrocolorimeter at a thickness of the light-absorbing solution layer of 10 mm and at a wavelength of 490-505 nm. Reference solution - distilled water.
Based on the data obtained, a calibration graph is built, plotting the mass of chlorine in micrograms on the abscissa axis, and the corresponding value of optical densities on the ordinate axis.
The calibration curve is checked once a quarter, as well as when replacing reagents or instruments.
It is allowed to calibrate the instrument using the least squares method.
Conducting an analysis
15 cm 3 of distilled water are placed in a volumetric flask with a capacity of 25 cm 3, stirring vigorously, 2 cm 3 of a solution of methyl orange, (0.5-2) cm 3 of the analyzed hydrochloric acid are injected with a pipette, the volume of the solution is adjusted to the mark with water and mixed. The optical density of the resulting solution is measured on a photoelectric colorimeter at a thickness of the light-absorbing solution layer of 10 mm and a wavelength of 490-505 nm. Reference solution - distilled water.
The mass of chlorine in micrograms in acid is found according to the calibration curve.
Results processing
Mass fraction of free chlorine X 3 , %, calculated by the formula:
Where m - the mass of chlorine in the analyzed hydrochloric acid, found from the calibration graph, mkt;
V - the volume of hydrochloric acid taken to perform the analysis, cm 3;
p - density of the analyzed hydrochloric acid, g/cm 3 .
The result of the analysis is taken as the arithmetic mean of the results of three parallel measurements, the discrepancies between which should not exceed 0.0003%, The results of the determination are rounded up to 0.0001%.
Permissible discrepancies between the results obtained in the two laboratories should not exceed 0.0005%.
The absolute total error of the determination is in the range of ±0.2 A, where A is the result of the determination at a confidence level P = 0.95
.
5.3 Transport and storage
The weight of the package must not exceed the carrying capacity of the pallet.
In a railway car, the packages are installed so that the capacity (carrying capacity) of the car is fully used.
Technical synthetic hydrochloric acid is stored in sealed containers of the manufacturer and consumer, made of materials resistant to hydrochloric acid.
The shelf life of the product is unlimited.
Conclusion
At the end of the course work, some conclusions can be drawn.
Hydrochloric acid is a clear, colorless or yellowish liquid without suspended or emulsified particles.
Hydrochloric acid is used in the chemical, medical, food industry, non-ferrous and ferrous metallurgy.
Hydrochloric acid (hydrochloric acid), whose chemical composition corresponds to hydrogen chloride, is widely used in a number of sectors of the national economy.
In industry, hydrochloric acid is produced in the following ways:
sulfate;
Synthetic
From off-gases (side gases) of a number of processes.
Technical synthetic hydrochloric acid must be produced in accordance with the requirements of the standards according to the technological regulations approved in the prescribed manner.
Technical synthetic hydrochloric acid in accordance with the rules for the transport of dangerous goods is transported:
In bulk in railway tanks ("Regulations for the Transport of Dangerous Goods No. 340", part 2, section 41);
Packed in barrels and bottles in boxes - by rail in covered wagons by wagon shipments ("Regulations for the Transport of Dangerous Goods No. 340", Part 2, Section 42);
Packed in containers, barrels, bottles - by road and water transport.
Barrels and bottles, when shipped in packages, are formed on flat wooden pallets in accordance with GOST 9557-87 in accordance with the requirements of GOST 21650, GOST 24597 and GOST 26663.
List SOURCES USED
1. Asaturyan N.G., Gol V.N. Reference book of non-food goods merchandiser. M.: Economics, 1990. - 349 p.
2. Brozovsky D.I. Merchandising of non-food products. M.: UNITI, 1990. - 398 p.
3. Demidova G.A. and others. "Commodity research of non-food products", V. 4, M. 1987.
4. Demidova G.A. Merchandising of non-food products. M.: Luch, 2000.- 487 s
5. Epifantseva V.V. Chemical industry of Belarus, Minsk: Higher school, 2005. - 274 p.
6. Karolkova R.V. Chemical industry, - St. Petersburg: Peter, 2005. - 285p.
7. Lifits I.M. Fundamentals of standardization, metrology, certification: Textbook. – M.: Yurayt, 1999. – 252 p.
8. Decree of the Committee for Standardization, Metrology and Certification under the Council of Ministers of the Republic of Belarus No. 35 dated 30.06.2004
9. Sytsko V.E., Drozd M.I. Commodity research of non-food products. Minsk: Higher school, 2005. - 663 p.
10. Economic newspaper - No. 12 - 2005
Nitric acid- monobasic acid, which has the form of a liquid with a yellowish tint. It is mainly used as a reagent, but there are many areas in which acid has also found use. The substance has a sharp unpleasant odor. Nitric acid belongs to substances of the 3rd hazard class. Therefore, storage and use of the reagent must be taken seriously. Nitric acid has the ability to oxidize most metals. Working with acid requires specialized clothing: overalls, shoes, gloves, respirator, goggles. When working with acid, you should be very careful, because contact of the substance with the skin can lead to unpleasant sensations and even allergies.
The use of nitric acid in industry
Due to its properties, the areas of application of nitric acid are very diverse:
- The chemical industry often resorts to the help of nitric acid. Artificial fiber is synthesized due to the participation in the process of nitric acid.
- The rocket and space industry uses acid to create rocket fuel.
- Jewelry is checked for authenticity with nitric acid. With the help of this chemical gold sample is determined.
- The metallurgical industry also uses nitric acid, the use of which guarantees the dissolution and etching of almost all known metals.
Many processes known to you involve nitric acid, the use of which in industry is of particular importance.
The use of nitric acid in everyday life
It is possible to clean jewelry at home. For this, nitric acid is used. Domestic use must be extremely careful to prevent the possibility of acid interaction with the skin. If it happens that the acid still gets on the skin, it is worth rinsing the affected area with tap water. Next, you can neutralize the acid with ammonia. Nitric acid in drip irrigation acts as a cleaner. To dissolve the sediment, or get rid of the accumulation of salts in the drip system, it is necessary to use 60% nitric acid.
The use of nitric acid in medicine
Some medicines contain nitric acid. The use of a chemical in medicine helps fight diseases of the skin and gastrointestinal tract. So, 30% nitric acid is used to combat the resulting warts. Acid concentration is of particular importance when we are talking about human health. For the treatment of peptic ulcer, nitric acid is used in the form of bismuth salts. This remedy has astringent and antiseptic properties.
The use of nitric acid in agriculture
In order for the harvest to be rich, many agronomists use mineral fertilizers. Some of them contain nitric acid. Application in agriculture is possible in the form of fertilizer or saltpeter. So that vegetables and fruits that have been exposed to fertilizer do not harm the health of the person who consumes them, it is necessary to clearly calculate the dose of fertilizer. An excess of the latter can lead to increased accumulation of nitrates in the final product. There are three types of nitrogen fertilizers: ammonia, nitrate and amide. Fertilizers tend to wash out on sandy soils.
In addition to nitric acid in its pure form, its derivatives have found use - salts of nitric acid, the use of which is in greater demand than their source. Some types of nitroesters are used in pharmaceutical preparations for the treatment of angina pectoris. Nitroglycerin is taken by people who suffer from coronary insufficiency.
The preparation and use of nitric acid requires special care. Therefore, it should be stored in rooms where there is a ventilation system. To avoid unpleasant situations, the container with nitric acid should be kept away from heating objects. Contact with light from the sun is also undesirable. Acid must be transported in hermetically sealed steel containers. Aluminum containers are used for storage.
You can buy nitric acid in the required quantity in our specialized store. Click the link to view the product



